Increased Blood Glucose Leads to Increased Sleepiness and Heart Rate
Without Change to Autonomic Tone
Derek Randolph, Georgia Gwinnett College
Shelby Pressler, Oglethorpe University
James B. Crabbe,* Georgia Gwinnett College
Abstract
Pre- and post-prandial measures were completed on 6 healthy individuals (2 men, 4 women, mean age 21 yrs.) to investigate the changes that a meal would have on blood glucose (mg/dL), feelings of sleepiness (VAS), and autonomic tone (SDRR; HRV). These measures were counterbalanced and compared to a no meal condition with the same participants. Glucose, sleepiness, and HR were significantly higher after a meal, while heart rate variability (HRV) was not significantly different between the protocols. The results of this study did not confirm the hypothesis that postprandial sleepiness would be related to adjustments in autonomic tone as measured by HRV.
Introduction
Sleepiness after a meal is a common experience that is not well understood. Various studies have observed the relationship between food and the possible causes of sleepiness (Orr, Shadid, Harnish, & Elsenbruch, 1997; Spring, Chiodo, & Bowen, 1987). There is common belief that after a meal insulin surges leading to glucose storage, which is accurate and supported by ubiquitous research (Brotman & Girod, 2002; Krentz, Viljoen, & Sinclair, 2013; Nilsson, Radeborg, & Bjorck, 2012). However, there is also a belief that this surge in insulin may cause a dip in blood glucose that may be involved in increased feelings of sleepiness or faster sleep onset latency. Although this may be a common belief, we found no good research to support this claim. It is unsubstantiated. Carbohydrate (CHO)-rich foods have the fastest and most significant impact on blood glucose concentrations. Although protein-rich foods have little effect on glucose concentrations, fat-rich foods have a delayed glucose response (Franz, 1997). The rapid rise in glucose from high-CHO foods results in a high insulin level response, which in turn causes the swift removal of glucose from the blood. The study of the influence of CHO on sleepiness by Wells, Read, Uvnas-Moberg, & Alster (1997) used 18 healthy individuals who consumed either a high-fat-low-CHO or low-fat-high-CHO meal. The study showed that levels of insulin and glucose were higher after the low-fat-high CHO meal, and increased insulin levels were positively correlated with sleepiness.
Postprandial sleepiness may also be attributed to the enhanced parasympathetic nervous system (PNS) drive necessary for gastric motility (Harnish, Greenleaf, & Orr, 1998). As a result of autonomic nervous system (ANS) activity, heart rate (HR) is directly affected by increasing or decreasing. The frequency with which HR increases or decreases is commonly known as heart rate variability (HRV) and can be used as a measure for autonomic nervous system (ANS) balance (Ambarish, Barde, Vyas, & Deepak, 2005; Landsberg, 2006; Tentolouris, et al., 2003). One typically used measure of HRV is the mean standard deviation of milliseconds between R waves of an ECG over a specified period of time (Karim, Hasan, & Ali, 2011). This is commonly referred to as SDRR (standard deviation of R to R). This change in autonomic tone may be a potential source of postprandial sleepiness; sleep onset is accompanied by increased parasympathetic nervous system activity (Burgess, Trinder, & Kim, 1996), which has been shown to be related to an increase in SDRR (Karim, Hasan, & Ali, 2011). Studies examining the effects of a meal on HRV are limited. A study using 9 healthy participants looked at the effects that a CHO or lipid infusion had on resting energy expenditure, HR, sleepiness, and mood (Wells, Read, & Macdonald, 1998). The result showed HR was significantly greater 3.5 hrs. after lipid and CHO infusions when compared to a control. CHO caused a rapid increase in HR, while lipids had a lesser and more delayed effect on HR. The participants were significantly sleepier 3 to 3.5 hrs. after the lipid infusion when compared to the control. Another study examining HRV pre- and post-prandial showed that HRV may not be significantly altered by meals in normal individuals (Ambarish, Barde, Vyas, & Deepak, 2005).
The study of postprandial sleepiness and its relationship to ANS balance has not been well examined. The aim of this study was to explore the interaction of HR, blood glucose, sleepiness, and autonomic balance after a standard meal. It was hypothesized that changes in autonomic tone such as an increase in SDRR (indicating increased PNS drive) would be related to changes in sleepiness.
Methods
IRB Approval
Research was conducted as part of classroom/lab activities in Human Physiology at Oglethorpe University. Each of the participants volunteered. They were not coerced, and it was made clear that their grade did not depend upon participation. The following statement was read and signed by each of the volunteers:
This study will assess the relationship of changes in heart rate (HR) dynamics, feelings of sleepiness, and blood glucose from before to after a standardized meal. As such I will be asked to sit quietly while these variables are collected via electrodes (HR), a visual analog scale (sleepiness), and blood sampling with a lancet (blood glucose). Moreover, one of the conditions will be with a meal and one will be without a meal. I will not be aware of the condition until it is time for the meal. I agree to participate in both the meal and the no meal conditions.
Participants
Six participants (2 male and 4 female, see Table 1) volunteered from the Human Physiology class at Oglethorpe University, Atlanta, GA. None of the participants were diabetic. Each participant was put in a group with two (or three) other students in the class that functioned as the experimenters. The participants were instructed to refrain from eating, being physically active, sleeping, or consuming caffeine for at least two hours prior to the experimental trials. Participants also recorded their personal characteristics including age, gender, height, weight, menstrual status for females, prior week's stress and physical activity, physical fitness status, and the quality and quantity of the prior night's sleep.
Procedures
Each participant served as their own control with the independent variable being consumption (or no consumption) of a meal. The two conditions were presented in a partially blinded and counterbalanced fashion. The participants were unaware of their condition until the very beginning of their first data collection periods. The dependent variables that were tested in this study were heart rate (HR), heart rate variability (HRV), sleepiness, and blood glucose concentration. The same procedure was carried out on two days separated by at least 24 hrs. at relatively the same time of day. Throughout the data collection periods, participants avoided sleeping as well as engaging in stimulatory activities (e.g., no cell phone use or participation in discussions). If the participant had to use the restroom, this was noted.
Test Meal
The participant's initial sleepiness, HR, and glucose level were recorded at 0 and 15 minutes. On the day when the participant received a meal, the meal was consumed between twenty and thirty minutes after the study began (time 0), and they were instructed to eat as much as possible during this time. The meal consisted of a 16.9 oz. bottle of water, a small chocolate chip cookie (~22.1g), macaroni and cheese (~198.3g), boiled greens (~88.1g), strawberries (~186.9g), and chicken tenders (~218.3g). For the remainder of this study, the participant's sleepiness, HR, and glucose levels were measured and recorded at 35, 50, 65, 80, and 95 minutes. This resulted in a total of seven sets of data for each participant and each condition.
Blood Glucose Concentration
Blood glucose levels were determined by using a handheld TRUEresult ® blood glucose reader. The participant's finger was cleaned using an alcohol swab and then pricked using a lancet. The blood drop was then delivered to a TRUEtest ® blood glucose test strip in the machine, and the machine provided the glucose levels in mg/dL.
Sleepiness VAS
For determining sleepiness, participants were asked to indicate their own level of sleepiness based upon a self-report visual analog scale (VAS) of 0 (very alert) to 200 (very sleepy) mm at each of the seven times that data was collected. The VAS has been used by many other experiments where sleepiness was studied (Short, Lack, & Wright , 2010; Tremaine, et al., 2010). VAS has shown to be a valid method of measuring psychosocial factors and is as effective, if not better than, the Stanford Sleepiness Scale when responding to the time of day and sleep deprivation (Hasson & Arnetz, 2005; Johns, 1991).
Heart Rate
HR was collected by ECG for two minute periods at each of the data collection periods using PowerLab/4ST and LabChart 7. A positive electrode was placed on the right side of the upper-chest area, a negative electrode was placed on the left side of the upper-chest area, and a ground electrode was placed below the negative one on the abdomen. The seven data sets collected were analyzed, and 120 R-R measurements were taken as a measure of HR for each.
Heart Rate Variability
HRV was calculated using the HR data and Microsoft Excel. The mean and standard deviation of the R-R intervals (SDRR) were calculated on Excel, and the SD was used here as a representation of HRV (Bray, 2000; Nagai, Sakane, Hamada, Kimura, & Moritani, 2005; Welle, 1995).
Analyses
Each of the dependent variables (glucose, sleepiness, HR, and HRV) was converted to a change score (post score – pre score at time 15) and analyzed with an RM ANOVA. Because of the low power of the study (N=6) and its experimental nature, statistical significance was set at p=.20. In addition, estimates of effect size are reported (η2).
Results
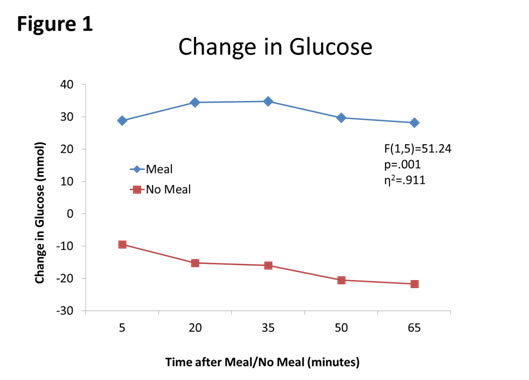
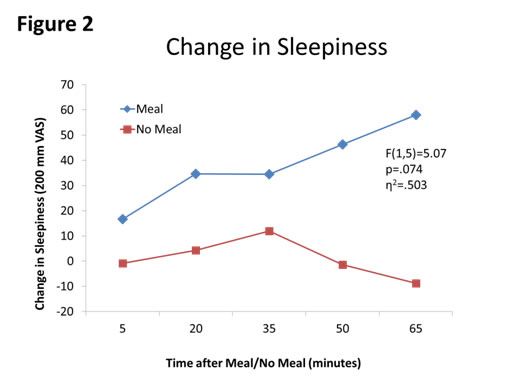
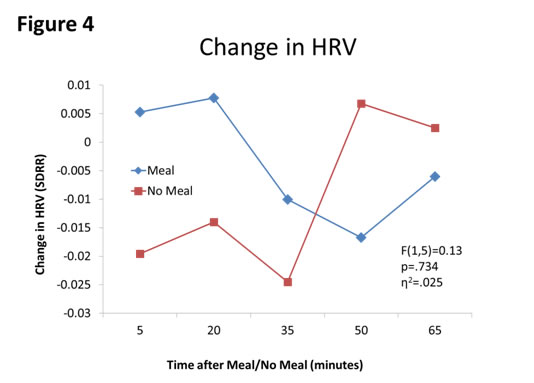
First, meal affected blood glucose concentrations (F(1,5)=51.24, p=.001, partial η2=.911, see Figure 1). Second, sleepiness was affected by meal moderately (F(1,5)=5.07, p=.074, η2=.503, see Figure 2). Third, HR was affected by meal (F(1,5)=2.43, p=.180, η2=.327, see Figure 3. Finally, autonomic tone (HRV) was not differentially affected by meal (F(1,5)=0.13, p=.734, η2=.025, see Figure 4).
Figure 1. Changes in blood glucose concentration (BGC) between Meal and No Meal conditions. BGC was higher at all points after the meal compared to the no meal condition.
Figure 2: Changes in Sleepiness between Meal and No Meal conditions. The meal moderately increased sleepiness after the meal compared to no meal.
Figure 3. Changes in HR (bpm) between Meal and No Meal conditions. HR increased after the meal compared to with no meal.
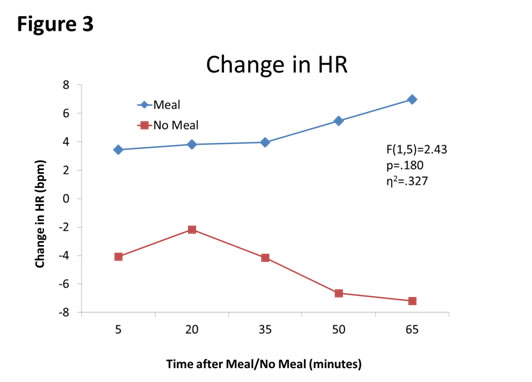
Figure 4. Changes in HRV between Meal and No Meal conditions. HRV was not affected by the meal compared to the no meal condition.
Discussion
This study examined the relationship between HR, glucose, sleepiness, and ANS balance after a meal. The meal induced significant changes in blood glucose concentrations when compared to the no meal protocol. This finding is similar to what has been found in other studies (Hlebowicz, Lindstedt, Bjorgell, & Dencker, 2011; Maki, Kanter, Rains, Hess, & Geohas, 2009; Nilsson, Radeborg, & Bjorck, 2012; Raben, et al., 2011). Wells et al. (1997) showed significant changes in plasma insulin and glucose concentrations after low-fat-high-CHO meals. These findings are consistent with other literature on meal consumption and changes in blood glucose concentrations (Benton, 2002).
The meal had a moderately significant effect on sleepiness when compared with the no meal condition. Although this study had similar effects on postprandial sleepiness that has been seen in other studies (Boelsma, Brink, Stafleu, & Hendriks, 2010; Orr, Shadid, Harnish, & Elsenbruch, 1997; Wells, Read, Idzikowski, & Jones, 1998), the quick onset of sleepiness seen between 50-65 minutes is much sooner than other studies have shown. Wells et al. (1997) showed significantly greater sleepiness 2-3 hrs. after high-fat-low-CHO meal. In another study by Wells, Read, & McDonald (1998) sleepiness was seen 3-3.5 h after lipid infusion when compared to saline infusion. Other literature suggests that feelings of less energy after high-CHO meal start about 2 hrs. after eating (Benton, 2002). Literature suggests that if our study had extended to 2-3 hrs. that a more significant change in the feelings of sleepiness may have occurred. Sleepiness may have also been affected by the prior night's sleep and sleep quality. A post-hoc analysis showed a negative correlation between the prior night's sleep quality with post meal feelings of sleepiness (-.50 to -.95) but no meaningful pattern emerged in the no meal condition. Future studies should control for the amount and quality of sleep of the participants.
HR was affected by the meal and remained higher at all-time points when compared with the no meal situation. Our finding was not completely consistent with other research in this area. Other studies show statistically different changes in HR 2-3.5 hrs. after a meal was consumed (Goldstein & Sharpiro, 1996; Sauder, Johnston, Skulas-Ray, Campbell, & West, 2012; Wells, Read, & Macdonald, 1998). Harthoorn & Dransfield (2008) showed HR increased by 10 percent immediately after meal for about 20 min., then decreased for the following 30 min. Forty-five to 50 minutes after meal a second prolonged increase in HR was observed for at least 1 hr.
Changes in autonomic tone as measured by HRV were not different between conditions. The changes in HRV after a meal were similar to results seen in other studies. Sauder, Johnston, Skulas-Ray, Campbell, West (2012) found that meal content had no effect on HRV under resting conditions. Millis et al. (2009) also showed that low/high frequency HRV spectral power was not significant after eliminating covariance of respiratory quotient, resting energy expenditure and HR for the effects of the control vs. high-fat treatment and for the high-fat vs isocaloric high-CHO treatment. Harthoorn & Dransfield (2009) also found that low/high frequency ratio were not significantly different from the pre-prandial level.
One limitation of this study was that the participants could not be fully blinded. They were all aware of the purpose of this study and what would occur in the second data collection period. This may or may not have attributed to the results found in this study, especially for sleepiness ratings. This study was also limited by the number of participants, which limited statistical power (remedied in part by the RM design) and therefore confidence in generalizing the outcomes. Another limitation was that the environment could have been better controlled to eliminate influence of outside variables. A chamber where the participant could be alone with no interaction with other people and limited amount stimuli would have been ideal for this study. Lastly, if the time points had been extended for 1-2 hrs. more, the data set may have been more complete. Due to the time restraints of the study and the participants, this was not a feasible procedure.
In conclusion, this study showed meal consumption caused changes in glucose, sleepiness, and HR. The hypothesis that postprandial sleepiness would more closely be related to the changes in PNS drive than changes in glucose was not confirmed. The increased levels of glucose led to an increased sleepiness and was seen within one hour after meal consumption. Post-prandial increases in HR were observed without changes in autonomic tone. Meal consumption does induce sleepiness; however the cause of postprandial sleepiness requires more research.
Table 1. Participant Characteristics
Age Gender Height (inches) Weight (lbs) Stress level for prior week (1-10) Physical activity for past week (hr) Self-Described Fitness rating (1-10) 21 Female 61 122 8 0 6.5 21 Female 64 132 8.5 2 4 21 Female 65 142 7 0 6 22 Female 67 137 5 7 7 21 Male 66 125 5 8 6 20 Male 70 188 7 6 6
References
Ambarish, V., Barde, P., Vyas, A., & Deepak, K. K. (2005). Comparison between pre-prandial and post-prandial heart rate variability. Indian Journal of Physiology and Pharmacology, 49(4), 436-442.
Benton, D. (2002). Carbohydrate ingestion, blood glucose and mood. Neuroscience and Biobehavioral Reviews, 26, 293-308.
Boelsma, E., Brink, E. J., Stafleu, A., & Hendriks, H. F. (2010). Measures of postprandial wellness after single intake of two protein-carbohydrate meals. Appetite, 54, 456-464.
Bray, G. (2000). Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implicatioins. International Journal of Obesity, 24(2), S8-S17.
Brotman, D., & Girod, J. (2002, Dec). The metabolic syndrome: a tug-of-war with no winner. Cleveland Clinic Journal of Medicine, 69(12), 990-9.
Burgess, H., Trinder, J., & Kim, Y. (1996, June). Cardiac parasympathetic nervous system activity does not increase in anticipation of sleep. Journal of Sleep Research, 5(2), 83-9.
Franz, M. (1997, Nov-Dec). Protein: metabolism and effect on blood glucose levels. Diabetes Education, 23(6), 643-6, 648, 650-1.
Goldstein, I. B., & Sharpiro, D. (1996). Postprandial ambulatory blood pressure and heart rate effects in healthy elderly adults. International Journal of Psychophysiology, 21, 91-95.
Harnish, M. J., Greenleaf, S. R., & Orr, W. C. (1998). A comparision of feeding to cephalic stimulation on postprandial sleepiness. Physiology & Behavior, 64(1), 93-96.
Harthoorn, L. F., & Dransfield, E. (2008). Periprandial changes of the sympathetic-parasympathetic balance related to percieved satiety in humans. European Journal of Applied Physiology, 102, 601-608.
Hasson, D., & Arnetz, B. B. (2005). Validation and findings comparing VAS vs. Likert Scales for psychosocial measurements. International Electronic Journal of Health Education, 8, 178-192.
Hlebowicz, J., Lindstedt, S., Bjorgell, O., & Dencker, M. (2011). Relationship between postprandial changes in cardiac left ventricular function, glucose and insulin concentrations, gastric emptying, and satiety in healthy subjects. Nutrition Journal, 10(1), 26-33.
Johns, M. W. (1991). A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep, 14(6), 540-545.
Karim, N., Hasan, J. A., & Ali, S. S. (2011). Heart rate variability- a review. Journal of Basic and Applied Sciences, 7(1), 71-77.
Krentz, A., Viljoen, A., & Sinclair, A. (2013, May). Insulin resistance: a risk marker for disease and disability in the older person. Diabetic Medicine, 30(5), 535-548.
Landsberg, L. (2006). Feast or famine: the sympathetic nervous system response to nutrient intake. Cellular and Molecular neurobiology, 26(4-6), 497-508.
Maki, K. C., Kanter, M., Rains, T. M., Hess, S. P., & Geohas, J. (2009). Acute effects of low insulinemic sweeteners on postprandial insulin and glucose concentrations in obese men. International Journal of Food Sciences and Nutrition, 60, 48-55.
Millis, R. M., Austin, R. E., Bond, V., Faruque, M., Goring, K. L., Hickey, B. M., . . . DeMeersman, R. E. (2009). Effects of high-carbohydrate and high-fat dietary treatments on measures of heart rate variability and sympathovagal balance. Life Sciences, 85, 141-145.
Nagai, N., Sakane, N., Hamada, T., Kimura, T., & Moritani, T. (2005). The effect of a high-carbohydrate meal on postprandial thermogenesis and sympathetic nerbous system activity in boys with a recent onset of obesity. Metabolism Clinical and Experimental, 54, 430-438.
Nilsson, A., Radeborg, K., & Bjorck, I. (2012). Effects on cognitive performance of modulating the postprandial blood glucose profile at breakfast. European Journal of Clinical Nutrition, 66, 1039-1043.
Orr, W. C., Shadid, G., Harnish, M. J., & Elsenbruch, S. (1997). Meal composition and its effect on postprandial sleepiness. Physiology & Behavior, 62(4), 709-712.
Raben, A., Moller, B. K., Flint, A., Vasilaras, T. H., Moller, A. C., Holst, J. J., & Astrup, A. (2011). Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks' sucrose-rich diet compared to an artificially sweetented diet: a randolmised controlled trial. Food and Nutrition Research, 55. doi:10.3402/fnr.v55i0.5961
Sauder, K. A., Johnston, E. R., Skulas-Ray, A. C., Campbell, T. S., & West, S. G. (2012). Effect of meal content on heart rate variability and cardiovascular reactivity to mental stress. Psychophysiology, 49, 470-477.
Short, M., Lack, L., & Wright , H. (2010). Does subjective sleepiness predict objective sleep propensity? Subjective Sleepiness and Objective Sleep Propensity, 33(1), 123-129.
Spring, B., Chiodo, J., & Bowen, D. J. (1987). Carbohydrates, tryptophan, and behavior: a methodological review. Psychological Bulletin, 102(2), 234-256.
Tentolouris, N., Tsigos, C., Perea, D., Koukou, E., Kyriaki, D., Kitsou, E., . . . Katsilambros, N. (2003, November). Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism, 52(11), 1426-1432.
Tremaine, R., Dorrian, J., Lack, L., Lovato, N., Ferguson, S., Zhou, X., & Roach, G. (2010). The relationship between subjective and objective sleepiness and performance during a simulated night-shift with a nap countermeasure. Applied Ergonomics, 42, 52-61.
Welle, S. (1995). Sympathetic nervous system response to intake. The American Journal of Clinical Nutrition, 62(suppl), 118S-22S.
Wells, A. S., Read, N. W., Idzikowski, C., & Jones, J. (1998). Effect of meals on objective and subjective measures of daytime sleepiness. Journal of Applied Physiology, 84, 507-515.
Wells, A. S., Read, N. W., Uvnas-Moberg, K., & Alster, P. (1997). Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiology & Behavior, 61(5), 679-686.
Wells, A. S., Read, N., & Macdonald, I. (1998). Effects of carbohydrate and lipid on resting energy expenditure, heart rate, sleepiness, and mood. Physiology & Behavior, 63(4), 621-628.
All rights reserved by the Undergraduate Research Community.
|