Glucocorticoid Effect on Bone and Glucose
Levels in
Six-Month Old Female Rats
Andrea
Beaver
Oklahoma State University
Abstract
Glucocorticoids are among the most potent and widely used immunosuppressant drugs available but have many detrimental side effects; however, only limited data are available on intervention studies to prevent these life-long side effects from occurring. The purpose of this study was to test an experimental rat model mimicking the bone loss and hyperglycemia associated with glucocorticoid administration. A five-week treatment was compared to a control group. The bone loss in the glucocorticoid-treated rats was found to be significant in only a brief treatment; the serum parameters may reflect that the gradual onset of diabetes was beginning. This study has provided a model of bone loss associated with the glucocorticoid (prednisolone) administered that can be used to test interventions to inhibit the adverse effects of glucocorticoids.
Introduction
On September 21, 1948, compound E (cortisone) became the first glucocorticoid to be administered to a patient with rheumatoid arthritis. Since that time the anti-inflammatory and immunosuppressive actions of glucocorticoids have benefited patients suffering from lupus and chronic asthma, along with organ transplant recipients (Avioli 1984, Frauman 1996). Unfortunately, glucocorticoid therapy does have several undesirable side effects. These may include central obesity, hypertension, impaired wound healing, increased infection rates, and impaired growth in children (Frauman 1996).
However, of particular interest and concern are the detrimental effects of glucocorticoids on bone and on glucose levels. Research has indicated that the use of glucocorticoids results in osteopenia (bone loss) and hyperglycemia, which can eventually lead to osteoporosis and diabetes mellitus (Naghavi et al 1975, Ravina et al 1999, Wimalawansa et al 1997).
Current osteoporosis trends indicate that 10 million individuals in the United States already have osteoporosis, and more than 18 million are at risk for developing osteoporosis (National Osteoporosis Foundation 2001). In addition, recent reports from the American Diabetes Association state that approximately 15.7 million people or 5.9% of the United States population currently suffer from diabetes mellitus (American Diabetes Association 2001).
Glucocorticoids have been shown to accelerate bone loss, leading to osteopenia and osteoporosis and also to impair glucose tolerance, leading to or worsening diabetes mellitus (Wimalawansa et al 1997, Ravina et al 1999). Glucocorticoids are among the most potent and widely used immunosuppressant drugs available (Frauman 1996) and due to the side effects, are considered an enormous problem in clinical practice today (Wimalawansa et al 1997). However, only limited data are available on intervention studies to prevent these life-long side effects from occurring. The purpose of this study was to test an experimental rat model mimicking the bone loss and hyperglycemia associated with glucocorticoid (prednisolone) administration. This model will be utilized in testing interventions to ameliorate the adverse side effects of glucocorticoids.
Materials and Methods
Animals and Diet
Twenty, six-month old, female Sprague-Dawley (Harlan-Teklad, Indianapolis) rats were randomly assigned to groups of ten fed either 100 mg prednisolone/kg diet or control diet with no added prednisolone. Prednisolone and dosages were chosen based on Lingren�s and colleagues� model that concluded prednisolone- induced osteopenia occurs in rats in doses of 100, 50, or 20 mg per kg of diet. To ensure prednisolone-induced osteopenia 100mg per kg diet was chosen.
The rats were individually housed in an environmentally controlled laboratory at the institution�s Laboratory Animal Resource Center (LAR). Rats were maintained on 12:12 light/dark cycles and allowed free access to distilled water throughout the duration of the study. Guidelines for the ethical care and treatment of animals established by the Animal Care and Use Committee at the institution were followed.
Rats consumed their assigned diet for five weeks and had free access to deionized water. During this time, rats were weighed once a week. Throughout the duration of the study, one rat from the prednisolone group, and one rat from the control group died before completing the entire five weeks.
Necropsy
After five weeks, the rats were placed in metabolic cages twelve hours before necropsy where urine was collected. At the time of necropsy, fasting blood glucose concentrations were measured using a Bayer Dex Glucometer Diabetes Care System (Albertson's Pharmacy) on a drop of blood from the tip of the tail. the animals then received an oral glucose load (1g/kg body weight) and after two hours, their glucose level was again measured. animals were then anesthetized intraperitoneally with ketamine (100 mg/kg body weight) and sylazine (5 mg/kg body weight) and whole body scans were performed by Dual Energy X-ray absorptiometry (DEXA) (Hologic QDR 4500 A, Waltham, MA>). The animals were then exsanguinated from the abdominal aorta. Blood was allowed to clot, centrifuged, serum aliquots frozen at -20�C until analysis. Liver, spleen, and kidneys were removed and weighed. Femur, tibia, third, fourth, and fifth lumbar vertebra were collected from the animals, cleaned of adhering tissues, and frozen at -20�C for further analysis.
Bone Parameters
Bone mineral area and bone mineral content were performed by Dual Energy X-ray Absorptiometry (DEXA) and analyzed with the small animal software provided by the manufacturer.
Serum Clinical Chemistry
Insulin values were assayed with a rat insulin RIA Kit (Linco Cat. # RI-13K, St. Charles, Mo.) and 125Iodine was measured on a gamma counter. Glucose and fructosamine were analyzed using a commercially available kit from Roche Diagnostics (Sommerville, NJ). These tests were performed using the Cobas-Fara II Clinical Analyzer (Roche Diagnostics, Sommerville, NJ).
Statistical Analyses
Data analysis of means and standard errors of the mean were calculated using SAS (version 8.0, SAS Institute, Cary, NC). The generalized linear model procedure was used for analysis of variance. The significance level was set at p<0.05.
Results and Discussion
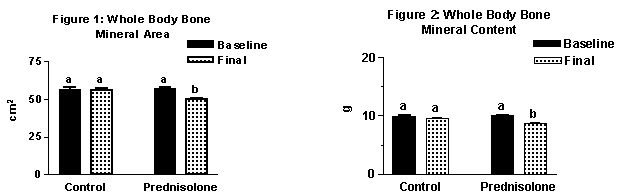
During the five-week treatment, the glucocorticoid group had a significant loss in body weight compared to the control group. The bone mineral area (Figure 1) and bone mineral content (Figure 2) of the glucocorticoid group were significantly lower in comparison to their basefline values. This was not observed in the control group. Apparently the bones of animals in the glucocorticoid-treated group decreased in size as well as in total mineral content.
|
|
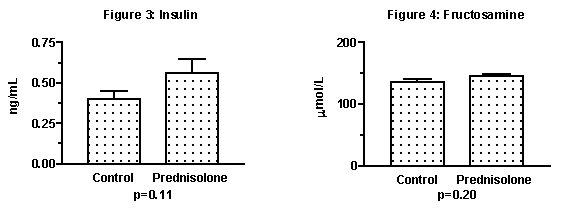
There were no significant differences found between the two groups for the blood parameters measured. The blood glucose concentrations did not indicate that diabetes mellitus had been induced in the rats. However, even during this relatively short experiment, the glucocorticoid treated group tended to have increase in their insulin (Figure 3). Elevated insulin occurs prior to the onset of overt Type II diabetes in humans. Fructosamine had increased by eight percent, which was not significant (Figure 4). Fructosamine is a measure of glucose control over the previous two to three weeks and higher fructosamine values reflect a history of higher circulating glucose. Because fructosamine changes gradually a longer study might be required to detect glucose changes that could be very important over a lifetime.Summary
Glucocorticoids are among the most potent and widely used immunosuppressant drugs available but have many detrimental side effects. However, only limited data are available on intervention studies to prevent these life-long side effects from occurring. Therefore, an effective rat model would aid in the effort to reduce negative treatment effects in humans associated with glucocorticoid treatment.
The bone loss in the glucocorticoid-treated rats was found to be significant in only a brief five-week period. In addition, the serum parameters may reflect that the gradual onset of diabetes was beginning. This study has provided a model of bone loss associated with the glucocorticoid (prednisolone) administered that can be used to test interventions to inhibit the adverse effects of glucocorticoids.
References
American Diabetes Association. Retrieved May 1, 2001, from http://www.diabetes.org/ada/cont.asp.
Avioli, L. V. (1984). Effects of chronic corticosteroid therapy on mineral metabolism and calcium absorption. Advances in Experimental Medicine and Biology, 171, 81-89.
Frauman, A. G. (1996). An overview of the adverse reactions to adrenal corticosteroids.
Adverse Drug Reactions Toxicology Review, 15(4), 203-206.
Mahan, K. L., & Escott-Stump, S. (1996). Food, Nutrition, and Diet Therapy, 9th Ed. Philadelphia: W. B. Saunders Co.
Naghavi, M., & Mesgarzadeh, A. (1975). On attempt to establish a model on steroid-induced osteoporosis in bones of rats. ACTA MEDICA IRANIC, 18, 175-193.
National Osteoporosis Foundation. Retrieved May 1, 2001, from http://www.nof.org.
Ravina, A., Slezak, L., Mirsky, N., Bryden, N. A., & Anderson, R. A. (1999). Reversal of corticosteroid-induced diabetes mellitis with supplemental chromium. Diabetic Medicine, 16, 164-167.
Wimalawansa S. J., Chapa M. T., Yallampalli, C.; Zhang, R., & Simmons, D. J. (1997). Prevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male rats. Bone, 21, 275-280.
|
|
|
©2002-2021 All rights reserved by the Undergraduate Research Community. |