Postnatal Environmental Influences on
Rat Dam Maternal Behavior and Offspring Anxiety
John M. Preslik
University of California, Berkeley
Abstract
Variations in maternal care are associated with the development of individual differences in behavioral and hypothalamic-pituitary-adrenal responses to stress in the rat. Environmental stressors can influence maternal care, and hidden variables can be problematic for research in behavioral science. When executing experiments involving animal models, the animal’s environment can have a dramatic influence on the results. We demonstrated how variations in bedding could lead to significant differences in maternal care and abnormal anxiety, e.g., behavior in their offspring that persists throughout adulthood.
Introduction
Hidden variables can be problematic for every application of science. When investigating mechanism’s for the nongenomic transmission of individual differences in stress reactivity across generations it's becoming increasingly evident that environment can have significant influences on the outcome (Plotsky & Meaney, 1993). The type of bedding used in animal models constitutes a large portion of the animal’s environment and can play a critical role in its development (Ivy et al., 2008).
The interaction that young pups receive from their mothers during the first week of life is crucial for appropriate maturation of the stress response and the underlying hypothalamus-pituitary adrenal (HPA) axis (Hess et al., 1969; Meaney & Aitken, 1985; Plotsky & Meaney, 1993). Adaptation of an organism to the environment occurs through numerous processes beginning in the prenatal period and continuing through the neonatal and early adolescent period (Ben-Ari, 1995; Katz & Shatz, 1996; Kolb & Whishaw, 1998; Wheal et al., 1998). In primates, rats, and humans, reduced levels of maternal care, either in the form of complete maternal deprivation or neglect, have been shown to increase stress responses, impair cognitive ability, and reduce social behavior (Arling & Harlow, 1967; Carlson & Earls, 1997; Glaser, 2000; Harlow, Dodsworth, & Harlow, 1965; Trickett & McBride-Chang, 1995). Enhanced maternal care increases the ability of the pups to deal with future stressors throughout life (Liu et al., 1997; Avishai-Eliner et al., 2001a; Fenoglio et al., 2006a) reflected in relatively reduced neuroendocrine stress responses and greater exploratory behavior in rats. In contrast, deficient maternal care promotes exaggerated stress responses later in life and less exploratory behavior (Schmidt et al., 2004). These data suggest that both the quantity and quality of the dam’s behavior and care critically influence the developing HPA axis, as well as shape the direction of its adult phenotype (O’Reagan et al., 2001; Welberg & Seckl, 2001; Avishai-Eliner et al., 2002; Fenoglio et al., 2006a,b).
The quality of maternal care provided by a dam can vary naturally from one individual to another. Such naturally occurring variations in maternal behavior are associated with the development of individual differences in behavioral and HPA responses to stress in the offspring. As adults, the offspring of high licking and grooming and arch-back nursing mothers are less fearful and show more modest HPA responses to stress than the offspring of low licking and grooming and arch-back nursing mothers (Stern, 1997; Caldji, 1998; Francis, Diorio, Liu, & Meany, 1999).
A common bedding material used to house animals for behavioral research is Tek-Fresh. Tek-Fresh (Fig. 4A) is composed of reclaimed virgin wood pulp and resembles crumbled pieces of brown paper. It is easily crafted into nests by dams’ before parturition. Purelite (Fig. 4B) is composed of ground corncob and has the texture of large kernelled sand. We proposed that using Purelite as a bedding substrate would alter the neonatal environment and influence the quality of maternal care and the developmental environment of the pups, therefore significantly changing the behavior of their offspring compared to their cohorts raised on Tek-Fresh. Here we describe the alterations of dams’ nurturing activities based on their bedding and the anxiety like behavior exhibited by their offspring.
A. B. Figure 1: (A) Tek-Fresh cage and bedding, (B) Purelite cage and bedding
Experimental Procedures
Animals
Long-Evans female rats were mated and housed in the federally approved University of California, Berkeley life science addition animal facility. Dams were maintained in quiet, temperature controlled rooms on a 12-hour light/dark cycle (6:30 pm lights off / 6:30 am lights on) with access to unlimited laboratory chow and water. Parturition was verified at 12-hour intervals (date of birth = day 0). On postnatal day 21 the male pups were weaned and female pups along with their mothers were culled. The male offspring were housed in groups of 2 according to the prenatal treatment until testing at 60 days of age.
A total of 15 dams with litters were utilized for experiments, and all rats were euthanized and rapidly decapitated to minimize suffering. All experiments were carried out according to NIH guidelines and were approved by the Institutional Animal Care and Use Committee. A conservative number of rat dams were used to minimize the amount of offspring, and only male offspring were used for adult offspring assessment.
The control group used normally bedded cages using approximately .33 cubic feet of Tek-Fresh laboratory animal bedding while the experimental group was housed using .33 cubic feet of corn-based Purelite bedding. All litters were undisturbed except for weekly bedding changes. All dams were housed in cages using either Tek-Fresh or Purelite bedding beginning 5 days before parturition.
Assessment of maternal behaviors
The activities of postnatal dams were observed three times a day at one-hour intervals for a total of 3 hours of observations each day. Observation times were 6:30 am to 7:30 am, 12:00 pm to 1:00 pm and 6:30 pm to 7:30 pm. This allowed for 2 hours of day (lights on) observations and 1 hour of night (lights off) observations. An observer watched and recorded dams’ activities in real time every 60 seconds for 60 minutes. This occurred over 14 consecutive days starting on the day of parturition. Activities eligible for scoring were, dam licking or grooming any pup, dam carrying any pup, dam self-licking or grooming, dam eating or drinking, and dam nursing more than half the litter. Types of nursing postures were also distinguished, using the following criteria: arched-back nursing was identified when the dam was positioned over her pups with back exaggerated into the air and side nursing where the dam was on her side with pups attached to her nipples.
The litter of each dam was labeled as high, middle, or low depending on the degree of licking and grooming received. Offspring were identified as high if they received an amount of licking and grooming that was greater than 2 standard deviations above the mean. Offspring were identified as low if they received an amount of licking and grooming that was less than 2 standard deviations below the mean. Offspring were identified as middle if they fell in between these two thresholds.
Elevated plus maze, open field and light-dark box tests
To examine whether or not the dams and their offspring exhibited “anxiety-like” behaviors that may be associated with variation in bedding material and maternal care, dams and their adult offspring were evaluated using the elevated plus maze, open field, and light-dark box paradigms.
The elevated plus maze consisted of two open arms 7.62 cm x 76.2 cm and two enclosed arms 7.62 cm x 76.2 cm and was elevated 76.2 cm above the floor. The enclosed arms and the open arms faced each other on opposite sides. Entries (four-paw criterion) and time spent in enclosed and open arms were measured, together with open/total arm entry ratios. An entry occurred whenever the rats crossed from one arm to another with four paws, re-entries into the same arm from the central region not being counted. Animals were placed in the terminal end of the maze facing an enclosed arm, considered as enclosed time until an open arm was entered, at the beginning of each trial. Conversely, an entry into an open arm lasted until the rats entered into an enclosed arm. Each rat was allowed one 5-minute trial in the maze, and the maze was cleaned with 70 percent ethanol solution after each trial. Time spent occupying open arm segments relative to closed arm segments, compared with control offspring, were used as an index of anxiety.
The light-dark box consisted of two contiguous rectangular arenas (76 x 40 cm) connected by a 10 x 10 cm entrance. The top of the light chamber was open to room lighting with the floor and three sides of the compartment being completely translucent. The dark chamber had a removable opaque black top as well as four opaque black walls. Animals were placed into the dark chamber through the door and then released. Time spent in the dark and light compartments were measured in addition to any entry made out of or into the dark chamber. An entry occurred whenever the rats crossed from one compartment of the box to the other with four paws. Each rat was allowed one 5-minute trial in the light-dark box and the boxes were cleaned with (70 % ethanol solution) after each trial. Time spent occupying open arm segments relative to closed arm segments, compared with control offspring, were used as an index of anxiety.
The open field paradigm utilized a large circular arena (140 cm in diameter, 61 cm in height) that was portioned into two areas. An inner arena was created (112 cm in diameter) and outer arena closer to the walls of the open field (14 cm in width). Duration of the time spent in the inner arena of the field and locomotion were measured in 5-minute trials.
All three tests were conducted in a quiet distraction-free room. The room was continually lit with a 60-watt light bulb attached to the center of the ceiling. All data were recorded live through a red filtered window located in the door of the testing room entrance and all tests were conducted between 11 am and 5 pm. Results were tabulated as a percent of total time in either the open arm, inner arena, or the light chamber.
Statistical analyses
All analyses were performed without knowledge of treatment group. Statistical significance was set at P<0.05. Unpaired Student’s t-tests were used for analysis of anxiety tests. The Prism GraphPad (San Diego, CA, USA) software package was utilized.
Results
Maternal Care
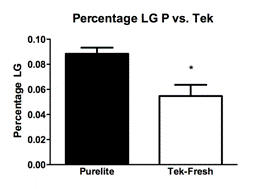
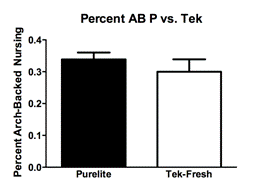
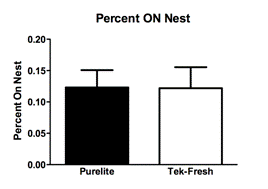
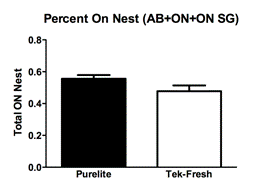
Observations of total licking and grooming on the pups by the mothers revealed a difference between the Purelite (Fig. 1A; Mean 0.08833 ± SEM 0.004917 N=4) and Tek-Fresh (Fig. 1A; Mean 0.05472 ± SEM 0.008893 N=4) mothers (R2=0.6458 P=0.0163). There were no observed differences of arched-back nursing on the pups by the mothers (Fig 1B; R2=0.1102 P=0.4217) between Purelite (Fig. 1B; Mean 0.3383 ± SEM 0.02185 N=4) and Tek-Fresh (Fig. 1B; Mean 0.2997 ± SEM 0.03909 N=4). There were no observed differences (Fig. 1C; P=0.9805) in time spent on the nest by mothers between the Purelite (Fig. 1C; Mean 0.1231 ± SEM 0.02776 N=4) and Tek-Fresh (Fig. 1C; Mean 0.1219 ± 0.03354 N=4) conditions. This was consistent with data comparing any behavior that indicated the mother’s presence on the nest. The behaviors that implied presence on the nest at the time of observation included arch-backed nursing (AB), being on the nest at the time of observation but not engaging in other activities (ON), and being on the nest while self grooming (ON SG). The total time on the nest including all relevant behaviors showed no difference (Fig 1D; R2=0.3567 P=0.1180) between the Purelite (Fig. 1D; Mean 0.5556 ± SEM 0.02319 N=4) and Tek-Fresh (Fig 1D; Mean 0.4778 ± SEM 0.03578 N=4) conditions.
A. B. C. D.
Figure 2: Maternal Behavior Displayed towards offspring.Light-dark box offspring
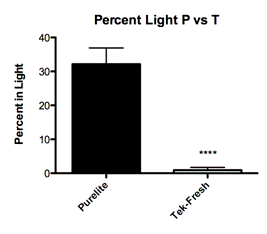
In adult offspring reared on Tek-Fresh bedding the rats occupied the light chamber for an average of .9 percent of the total trial time or 2.7 seconds (Fig. 2B; Mean=0.900% ± 0.79 N=10). The adult offspring reared on Purelite bedding occupied the light chamber for a mean of 29.45 percent of their total trial time or 88.35 seconds (Fig. 2B; Mean=29.45 ± 5.127 N=20). The means of these groups differed significantly (t=3.892) and it is unlikely that this was the result of chance (P=0.0006).
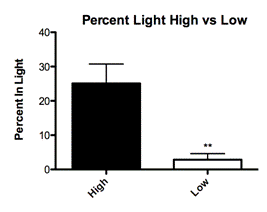
Offspring identified as having received low quality maternal care displayed significantly more anxious behavior (Fig. 2C; Mean 2.857 ± SEM 1.752 N=7), spending an average of 2.9 percent of each trial or 75 seconds in the light chamber compared to their high quality (High LG) counterparts (Fig. 2C; Mean 25.09 ± SEM 5.659 N=11) who spent an average of 25 percent of each trial or 8.7 seconds in the light chamber. This result was independent of their bedding condition and based solely on their licking and grooming scores.
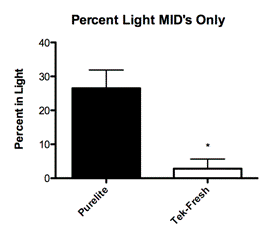
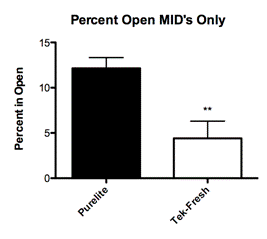
Eliminating all offspring that were categorized as having received High and Low levels of maternal care left a group that received the same amount of maternal care (mids) but were reared on different bedding conditions. The mids raised on the Purelite condition spent an average of 27 percent of total trial time exploring the light chamber (Fig. 2A; Mean 26.53 ± SEM 5.349 N=19) while the mids in the Tek-Fresh condition spent an average of 3 percent of total trial time exploring the light chamber (Fig. 2A; Mean 2.833 ± SEM 2.833 N=6)
A. B. C. Figure 3: Adult offspring performance in light-dark boxes by condition
Open Field
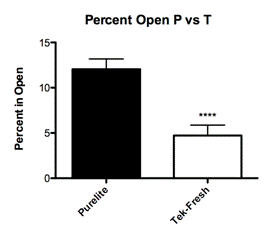
Adult offspring reared on Purelite spent an average 12 percent of total trial time exploring the center of the open field chamber (Fig. 3A; Mean 12.04 ± SEM 1.148 N=26) while the adult offspring reared on Tek-Fresh spent an average 5 percent of total trial time exploring the center (Fig. 3A; Mean 4.722 ± SEM 1.148 N=18). Offspring raised on Purelite spent significantly more time exploring (P< 0.0001) than their Tek-Fresh cohorts.
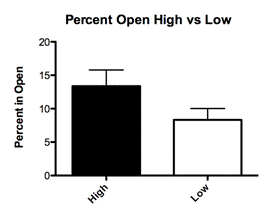
Adult offspring were also tested based on the amount of maternal care they had received regardless of the bedding type they were raised on. Offspring categorized as having received High maternal care spent an average of 13 percent of total trial time exploring the center of the field (Fig. 3C; Mean 13.38 ± SEM 2.420 N=8). Offspring categorized as having relieved Low maternal care spent an average of 8 percent of total trial time exploring the center of the open field chamber (Fig. 3C; Mean 8.333 ± SEM 1.694 N=12). The differences in these two groups were not significant (P=0.0949)
Eliminating all offspring that were categorized as having received High and Low levels of maternal care left a group that received the same amount of maternal care (mids) but were reared on different bedding conditions. The mids raised on Purelite spent an average of 12 percent of total trial time exploring the center (Fig. 3B; Mean 12.14 ± SEM 1.194 N=21) while the mids raised on Tek-Fresh spent an average of 4 percent of total trial time exploring the center (Fig. 3B; Mean 4.400 ± 1.910 N=10). The differences between these two groups were significant (P=0.0013).
Figure 4: Adult offspring performance in open field tests by condition
A. B. C. D. Discussion
Mothers who reared their young on Purelite provided higher levels of licking and grooming compared to their cohorts on Tek-Fresh. Increasing quantity and quality of maternal care, especially licking and grooming received by the pups, can mediate the expression of fearfulness on anxiety measures (Caldji, 1998). The adult offspring who received High levels of licking and grooming (2 standard deviations above the mean) displayed significantly more exploratory and fearless behavior in light-dark boxes (Fig. 2C; P=0.0077). In open field tests you can see a visual trend of increased exploratory behavior, but these results were not significant (Fig. 3C; P=0.0949). This behavior is what we would expect to see from individuals who received higher levels of maternal care (Liu, 1997).
When offspring reared on Purelite were examined, regardless of the level of maternal care, we found that the offspring behaved less anxious on both open field (Fig. 3A; P< 0.0001) and light-dark box measures (Fig. 2A; P=0.0006). This demonstrated a significant difference in adult behavior as a function of bedding material. The composition of this group encompassed the entire range of maternal care from low, mid, and high.
To further explore the effects of behavioral change in adult rats as a function of bedding material alone, we decided to control for the type of maternal care received by each litter. We proceeded to test only offspring that were classified as mid (licking and grooming between 2 standard deviations above and below the mean). The mids all received approximately the same amount of maternal care and differed only in their bedding. The mids reared on Purelite showed significantly less anxious exploratory behavior in open field trials (Fig. 3B; P=0.0013) and in light-dark box trials (Fig. 2A; P=0.0237). These results demonstrate that regardless of the quality or quantity of maternal care the offspring displayed significant behavioral differences as a function of bedding alone.
When employing animal models to pursue knowledge, hidden variables can be problematic. This is especially true when investigating nongenomic transmissions of individual differences in stress reactivity across generations. Environmental variables such as bedding should be considered an important influence across natal development. Changes in early developmental environments can have profound effects on the quality of maternal care (Plotsky & Meaney, 1993) in rat dams, and the results of natural variation in maternal care on offspring are well documented (Liu, 1997). This study has demonstrated that the bedding component of a rat’s environment can have a profound effect on offspring behavior regardless of the maternal care received, making it a critical consideration when drafting experimental designs and protocols.
References
Arling, G. L., Harlow, H. F. (1967). Effects of social deprivation on maternal behavior of rhesus monkies. Journal of Comparative & Physiological Psychology, 64, 371-377.
Avishai-Eliner, S., Brunson, K. L., Sandman, C. A., & Baram, T. Z. (2002). Stressed-out, or in (utero)? Trends in Neuroscience , 319-329.
Ben-Ari, Y. (1995). Activity-dependent forms of plasticity. Journal of Neurobiology , 295-298.
Caldji, C. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences , 5335-5340.
Carlson, M., & Earls, F. (1997). Psychological and neuroendocrinological consequences of early social deprivation in institutionalized children in Romania. Integrative Neurobiology of Affiliation. Annals of NY Academy of Sciences, 419-428.
Fenoglio, K. A., Brunson, K. L., & Baram, T. Z. (2006a). Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Frontiers in Neuroscience , 180-192.
Fenoglio, K. A., Chen, Y., & Baram, T. Z. (2006b). Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. Journal of Neuroscience , 2434-2442.
Francis, D., Diorio, J., Liu, D., & Meany, M. (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science , 1155-1158.
Glaser, R. (Ed.) (2000). Advances in instructional psychology educational design and cognitive Science, Vol. 5. Mahawah, NJ: Laurence Erlbaum and Associates.
Harlow, H. F. Dodsworth, R. O., & Harlow, M. K. (1965). Total isolation in monkeys. Proceedings of National Academy of Science, 54(1), 90-97.
Heim, C., Plotsky, P. M., & Nemeroff, C. B. (2004). Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology , 641-648.
Hess, J. L., Denenberg, V. H., Zarrow, M., & Peiffer, W. D. (1969). Modification of the corticosterone response curve as a function of handling in infancy. Physiology and Behavior , 102-109.
Ivy, A. S., Brunson, K. L., Sandman, C., & Baram, T. Z. (2008, June 26.). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience, 154 (3): 1132-1142.
Katz, L. C., & Shatz, C. J. (1996). Synaptic activity and the construction of cortical circuits. Science , 1133-1138.
Kolb, B., & Whishaw, L. Q. (1998). Brain plasticity and behavior. Annual Review Psychology , 43-64.
Liu, D. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothala- mic-pituitary-adrenal responses to stress. Science , 1659-1662.
Liu, D., Diorio, J., Tannenbaum, B., Caldji, C., Francis, D., & Freedman, A. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypo- thalamic-pituitary-adrenal responses to stress. Science , 1659-1662.
Meaney, M. J., & Aitken, D. H. (1985). The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations. Brain Research , 301-304.
O’Reagan, D., Welburg, L. L., Holmes, M. C., Seckl, J. R. (2001). Glucocorticoid programming of pituitary-adrenal function: mechanisms and physiological consequences. Semin Neonatal, 6, 319–329.
Plotsky, P. M., & Meaney, M. J. (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research , 195-200.
Schmidt, M., Enthoven, L., van Woezik, J. H., Levine, S., de Kloet, E. R. , & Oitzl, M. S. (2004). The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. Journal of Neuroendocrinol, 16:53–57.
Stern, J. M. (1997). Offspring-induced nurturance: animal-human parallels. Developmental Psychobiology , 19-37.
Trickett, P. K., & Mcbride-Chang, C., (1995). The developmental impact of different forms of child abuse and neglect. Developmental Review, Vol. 15, No. 3. , pp. 311-337.
Welberg, L. A., & Seckl, J. R. (2001) Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinol, 13(2):113–128.
Wheal, H. V., Chen, Y., Mitchell, J., Schachner, M., Maerz, W., Wieland, H., et al. (1998). Molecular mechanisms that underlie structural and functional changes at the postsynaptic membrane during synaptic plasticity. Progress in Neurobiology , 611-640.
|