Mitigating Osteolysis in Clinical Total Hip Arthroplasty:
The Role of Polyethylene Wear in Limiting the Life of Biomechanical Hip Implants
Sandhya Ravichandran
Case Western Reserve University
Abstract
This article critically examines the role of ultrahigh molecular weight polyethylene (UHMWPE) wear and the effect of wear particles in limiting the life and performance of hip implants used in Total Hip Anthroplasty. Polyethylene wear debris, generated at the bearing surfaces of hip prostheses, has been shown to have a direct link to osteolysis and implant loosening. Osteolysis is an inflammatory biological response caused by macrophages that engulf wear debris formed in the peri-prosthetic tissue. Osteolysis eventually leads to the loosening of an implant and inevitably leads to the surgical removal of the implant. Researchers have investigated several wear particle characteristics including particle size, volume, and shape and have shown that such characteristics are essential to understand the causes of osteolysis. Research also indicates that contact stress, the orientation of molecules in UHMWPE, and the roughness of the bearing surfaces are additional factors in affecting implant life. In relation to this, it has also been shown that the wear of UHMWPE is a function of implant loading and positioning. Research on electron-beam-crosslinked UHMWPE tested against various implant surface roughness demonstrates that cross-linking improves wear resistance dramatically. The general conclusion that can be derived is that understanding how to control particle wear is vital to prevent implant failure in Total Hip Anthroplasty. However, much research remains to be done to completely understand the role of UHMWPE particles in osteolysis resulting from total hip arthroplasty.
INTRODUCTION
Due to increases in life expectancy in the United States, the demand for implants continues to increase annually, especially among the elderly population. Within the next several years, the fraction of United States population older than fifty is expected to double, which means that there will be more elderly people in need for total hip anthroplasty. The demand for total joint replacement is becoming apparent among younger generations as well. It has been reported [1] that around 800,000 artificial joints are implanted every year. In addition to being one of the major successes in orthopedic medicine, Total Hip Arthroplasty (THA) has become one of the most widely employed surgical procedures available for joint replacement. Total Hip Arthroplasty is the medical procedure of replacing the complete hip joint with a mechanical system that consists of a femoral device articulating against an acetabular cup. Such a joint system is made in the form of metal on metal, metal on ceramic, or ceramic on ceramic articulations. A metal on ceramic device contains an interlayer of the ultra-high-molecular-weight-polyethylene (UHMWPE) polymer to facilitate lubrication and smooth articulation of the joint surfaces. The most popular joint system made of a Titanium cup lined with UHMWPE. Figure 1 illustrates the general design of the hip implant system and its assembly in the human body [2]. To understand how the system and assembly works, we need to understand the structure of the hip. Figure 1-a displays some basic components of the hip joint. The hip joint is like a ball that fits in a socket. The socket is called the acetabulum. The ball sits at the top of the femur, and thus is called the femoral head. The femur is the long bone that runs from the hip down through the thigh. In an artificial hip, the femoral head is on top of a femoral stem. The stem is inserted inside the femur bone, connecting the leg to the hip (seen in Figure 1-b).
1A. 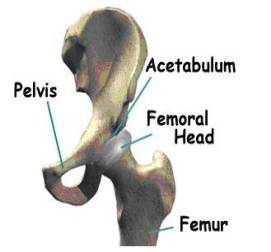 1B.
1B. 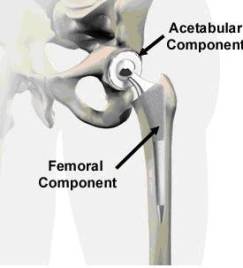
Figure 1. Anatomy of hip joints and components of the implant system in total hip arthroplasty. Figure 1 A-B were taken from Medical Multimedia group , www.jointreplacement.com [2].
Although the implanted devices greatly benefit thousands of patients every year, their longevity is limited by several material considerations. There are three main factors that limit total hip systems. The foremost among them is the wear of UHMWPE interlayer, aggravated by the complex relative motions between the femoral head and acetabular cup. One of the earliest polymer materials used before UHMWPE in joint replacement systems was polytetrafluoroethylene (PTFE). Figure 2 shows morphologies of worn UHMWPE of a total hip joint system made in 1958 [3]. In this design, the femoral head and the shell were press fit into the bone, with the idea that the friction between the bone and the PTFE would prevent relative motion. However, the tolerances between the head and shell were such that the articulation tended to occur mainly between the PFTE shell and the acetabulum, resulting in abrasion of the shell and destruction of the underlying bone. Wear also occurred of the PTFE femoral head prosthesis. Secondly, it has become apparent that loosening of implants is induced by the accumulation of wear debris in the body. The loosening limits the life expectancy of an artificial hip to anywhere from 15-25 years, which is too short considering the trauma and expense associated with any surgical revisions to correct the problem. Lastly, there have been several studies conducted to understand the wear generation of UHMWPE that are not covered in this paper. For example, how wear generation is a function of fluid volume (serum-bodily fluid), protein concentration and anatomical positioning is a vital area of research on this subject. Figure 3 combines the three areas of research to give an illustration of how the wear of UHMWPE and PTFE is affected by these environmental influences in the body and the loading of the implant [4]. The figure describes a study aimed to examine the fluid and volume effect on PTFE and the effect of positioning: inverted, anatomical horizontal and anatomical oblique on UHMWPE and PTFE. The study concluded that as volume decreased protein precipitate increased.
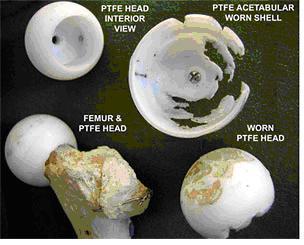
Figure 2 . Charnley's design of an artificial hip joint, and the wear of its PTFE interlayer over a period of 2 years. The photograph was obtained from www.uhmwpe.org, and the photograph was originally taken of the collection at the Charnley Museum at Wrightington [3].
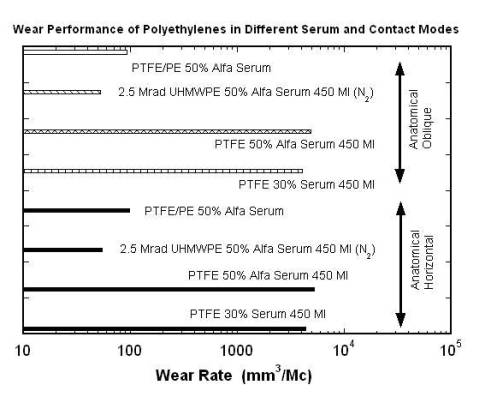
Figure 3 . The figure illustrates how polyethylene wear is related to anatomical positioning and environmental factors (serum.) Data was taken from: Good V., Clarke I., Gustafon A., Anissian L. Wear of UHMWPE and PTFE in a him simulator: comparison of fluid volume and protein concentration and anatomical vs. inverted positioning. In 47 th Annual Meeting, Orthopaedic Research Society, 2001, p. 1015 [4].
WHAT IS OSTEOLYSIS?
Osteolysis is a biological phenomenon that can result in the loosening of the implant, and it is principally caused by the UHMWPE wear particles as well as metal or ceramic wear particles that are produced at the articulating surfaces of a hip prosthesis. There is compelling evidence to suggest that osteolysis is influenced by the size and morphology of the UHMWPE particles [5,6]. Implant loosening is a result of the biological interaction between macrophages and wear particles. It is understood that macrophages, inflammatory cells within the body, actively phagocytose (engulf) wear debris at the bone-implant interface. These cells release various enzymes and osteolytic mediators such as interleukin, tumor necrosis factor (TNF-α), and prostaglandin, which are generally referred to as cytokines [6]. These cytokines cause inflammation and trigger bone dissolution or resorption around the implanted region. Through in vitro studies of the effect of particle size in bone resorption, researchers [1,7,8] have shown the importance of size and dose of clinically relevant polyethylene particles on the osteolytic activity of the macrophages. Recent studies have also focused on the effect of wear on osteolysis [9]. Furthermore, material modification approaches such as electron-beam-crosslinking seem to have the potential to reduce the wear of UHMWPE. Polyethylene is an organic compound formed by long repeating chains of a single substance: the molecules of the gaseous substance ethylene . In Figure 4, diagrams 4-A and 4-B show how UHMWPE is crosslinked and C presents how ethylene is polymerized to become polyethylene [10]. The mechanism (illustrated by Figure 4-A and 4-C) of cross-linking involves the following: The irradiation knocks an electron out of the Hydrogen atom. The hydrogen atoms then wander out of the polyethylene molecule as free radicals (blue balls with red hats in the picture). This happens simultaneously on several places in several UHMWPE; the carbon atoms that are lacking their hydrogen neighbors keep "free hands" out to find a new neighbor [10].
4A. 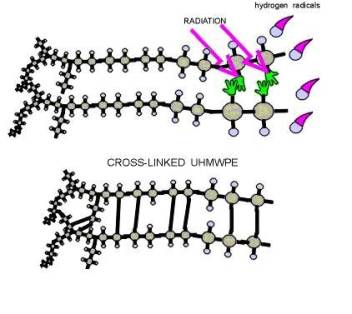
4B. 
4C. 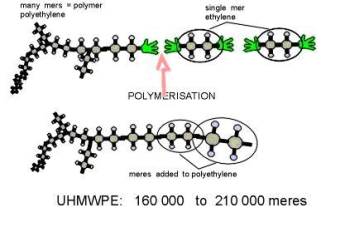
Figure 4. A and B illustrate how polyethylene is crosslinked, while C represents the polymerization of ethylene to form polyethylene. Diagrams were obtained from: Surin, Valdemar. “Polyethylene for Total Hips.” (10 Apr. 1999): 8pp. On-line. Internet .24 February 2003. Available FTP: http://www.totaljoints.info/polyethylene_for_total_hips.htm [10]
The goal of this review is to compile and critically assess the scientific and technical advances that have taken place in this area, and highlight some of the major directions of progress.
THE HISTORY OF PLASTIC WEAR OSTEOLYSIS IN IMPLANTS
The loosening of total hip implants, caused by polyethylene wear particles, was first observed by Charnley in 1968 [3, 11]. When analyzing cystic erosion in the proximal femur of the bone, he found evidence of osteolysis. The tissues contained a large number of macrophages caused by the accumulation of particles from polymethylmethacrylate (PMMA), a form of plastic that was commonly used in earlier implant designs and is currently used as bone cement for joint systems. Wilert and Semlitsch [12] proposed, on the basis of microscopic observations, that aseptic loosening resulted from the accumulation of wear debris seen within the periprosthetic membrane. They concluded that the wear debris from the plastic was the direct cause of osteolysis. Since then the experimental ability to observe, isolate, and study the effects of UHMWPE particles has increased significantly. The characterization of UHMWPE can be performed through careful in vivo studies. “In vivo” in this context refers to the study of osteolyzed tissue after explanting the implant, for example, using various methods of optical microscopy. It is now possible to routinely view UHMWPE in histological sections of the periprosthetic tissue using polarized light microscopy. Such in vivo histological studies of tissues indicate that small particles of less than 5 microns are embedded inside macrophages whereas relatively larger particles are associated with multi-nucleated foreign body giant cells [13]. The resolution of the light microscope limits observations of features of the order to generally one micrometer in size or more. This drawback led to interest in microscopic techniques of higher resolution. Recently, scanning electron microscopy (SEM) has been used to determine the size of the wear particles at magnifications higher than that of an optical microscope. In vivo study of UHMWPE induced osteolysis has been enhanced with the use of SEM.
In vitro study, which is done in an artificial environment and outside the body, has also been used as a supplement in the study of UHMWPE wear in general. “In vitro” generally refers to the study of interactions between given constituents in a standard laboratory environment. In vitro study allows for isolated analysis of UHMWPE particles, and the complications due to other variables inside the body can be avoided. However, such experiments lack the ability to monitor the effects of biological mechanisms, such as the interactions of organs, blood, and molecular components of the body with wear particles. Furthermore, polyethylene is difficult to use in vitro, because it is less dense than the culture medium and floats on the medium surface [14].
Although in vitro and in vivo research has advanced a great deal, there are issues to be resolved. For example, data obtained from in vitro studies can vary considerably from data obtained from in vivo study. For example, Elfick et al. [15], in their hip simulator tests using explanted femoral heads which had been roughened in vivo, observed that the in vitro wear rates were five times higher than the in vivo wear rates. Such discrepancies need to be resolved in order to ensure that in vitro results are applicable in vivo.
CHARACTERIZATION OF WEAR PARTICLES: APPROACHES, MATERIALS, METHODS, AND MAJOR FINDINGS
In biomedical research, there seems to be a consensus [1,6,7,15] that to reduce or eliminate the osteolysis caused by UHMWPE wear debris, three areas need to be targeted: (a) the examination of the role of interlayer stress/contact conditions, (b) a better understanding of the biological reactions of the body in response to wear debris and (c) the improvements to the molecular structure of UHMWPE to reduce wear. In the following, the approaches and specific experimental methods employed, as well as the major findings, are discussed.
Morphology of UHMWPE as a function of contact stress and orientation
Although it has been established that particle size and morphology play a significant role in biological responses, past studies [9, 11, 13] failed to address the specific geometrical features of particles that may be important. Research based on particle descriptors to analyze particles generated in vivo has emerged only recently. In a study by Landry et al. [6], the effect of three different parameters on the characteristics of UHMWPE particles were investigated: initial surface roughness of UHMWPE samples, orientation of the wear surface with respect to the UHMWPE bar, and contact stress. Six different particle descriptors were used to quantify particle shape. Equivalent Circle Diameter (ECD), a measure of overall particle size, was used as a measure of length. The quantification of particle shape was attempted using descriptors including Aspect Ratio (AR), Elongation (E), Roundness (R), Form Factor (FF), and Outline Fractal Dimension (OFD). AR corresponds to the ratio of the major diameter to the minor diameter, E refers to the length of long and thin particles, R varies from 0 to 1 (perfect circle), FF is based on the perimeter of the particle, and OFD is based on the irregularity of the surface. Two different initial roughness values varying between a smooth (R=1.0um) condition and a rough (R=3.5um) condition were used to determine the effect of UHMWPE surface roughness on the size and morphology of wear particles. Four specimens in each surface preparation method were tested using a unidirectional pin on disk rotational wear testing machine with four identical stations. The effect of contact stress between the bearing surfaces was studied using two different stress levels: low (6.9 MPa) and high (13.8 MPa). The effect of orientation, which refers to the angle at which the samples were taken from the UHMWPE bar stock, on wear particles was studied using the same cylindrical rotational wear machine. The three UHMWPE sample orientations employed were: 0 degrees, 45 degrees, and 90 degrees [6].
The mean (ECD) of the wear particles from smooth UHMWPE samples was lower compared to the rough samples. Wear particles less than 1.0 micron accounted for 83.6% of the debris generated in the smooth UHMWPE test sample, but accounted for only 70.4% of the debris generated in the rough sample. The values for aspect ratio, elongation, form factor, and outline fractual dimension between smooth and rough UHMWPE specimens were not significantly different between the smooth and rough test samples [6]. When contact stress was doubled from 6. 9 to 13.8 MPa, the mean size of UHMWPE wear particles increased from 0.48 to 0.91 micron. The contact stress also affected particle shape. In the low stress tests, particles were relatively smaller and rounder than that in the high stress tests. Results from the orientation tests indicated that the ECD and E values increased in tests with orientations from 0 degrees to 45 degrees, but decreased in tests with orientations from 45 degrees to 90 degrees [6]. It is interesting to note that the mean particle size for the 0 degrees and 90 degrees orientations was similar and lower than the 45 degrees specimens. The study concluded that it is possible that particle size is related to surface energy, which is influenced by molecular orientation at the surface. These results are vital to elucidate potential correlations between particle size and surface energy [6,16].
Although the study by Landry et al. established that, under controlled conditions, contact conditions and material parameters affect the size and morphology of wear debris, further research to determine the desirable particle shape characteristics that may reduce osteolysis is needed. Nevertheless, studies have shown the importance of the mechanical conditions and material parameters that affect particle characteristics during in vitro wear and encouraged the use of standardized size or shape descriptors, which make it easy to compare different studies and obtain conclusive results.
Effect of wear particle size and dose on bone resorption activity and the induction of cytokines
In an attempt to understand the importance of particle size in osteolysis, Fisher et al [1] studied the effect of particle size and dose on the release of cytokines in vitro. Interleukin (IL-6) and tumor necrosis factor (TNF-α) were two of the cytokines that were examined. The polyethylene particles were classified into various sizes and were tested to determine the “critical” size range for macrophage activation. Polyethylene particle sizes of 0.21, 0.49, 4.3, and 7.2 microns and a polyethylene resin with a mean particle size of 88 microns were used. All were co-cultured with C3H murine peritoneal macrophages to give cell number ratios of 100:1, 10:1, 1:1, and 0.1:1 [1]. Enzyme-linked immunosorbent assays (ELISA) were used to determine the production levels of cytokines (IL-6, TNF-α, etc.) It is important to note that previous studies [8,17] had failed to take into account the fact that polyethylene particles float in a culture medium, which limits the macrophages ability to phagocytose them. However, the second study performed [1] by Fisher et al two years after the one conducted in 1998 [8] optimizes the macrophage-particle interactions by encapsulating the polyethylene particles superficially in agarose gels and culturing the primary macrophages on top of the particle-agarose gel.
In the study above, bone resorption activity was measured through testing cytokines for the inflammatory activity level that each type of cytokine (Interleukin, TNF-α, etc.) produced. Interleukin activity was produced by 100:1 cell number ratios in the specific activity amounts of 2.41 and 3.15 of TNF-α activity. Peritoneal macrophages secreted 1.4 units. Polyethylene particles with a mean size of 0.49, 4.3, and 7.2 µm at 100:1 ratios produced 33.3, 35.58, and 16.46 units of TNF-α specific activity [1,8]. Therefore, the most biologically active particles were 0.49 and 4.3 µm in size with the smaller (0.21 µm) and larger (7.2 µm) particles being inactive at particle volume cubic microns to cell number ratios of 10:1 and 100:1. Particles outside these treatment groups did not produce significantly elevated levels of interleukin (cytokine) activity. When experiments were repeated [1,8] using the same particle size distributions at ratios of 10 and 100 cubic microns to one macrophage, the results showed the same cytokine production trends.
The study illustrates that UHMWPE particles can stimulate primary macrophages to produce elevated levels of osteolytic cytokines, IL-6 and TNF-α, in vitro. Based on the results, it can be confirmed that the size of particles is a critical factor in macrophage activation, with the most biologically active particles in the phagocytosable size range of 0.3 to 10 um [17]. The crucial information obtained from these experiments was that both the largest (88um) and the smallest (.21um) particles failed to stimulate the macrophages to produce significant amounts of cytokines [18]. The role for TNF-α in the osteolytic process was also demonstrated. The conclusions drawn by Green et al. allow for the prediction of the osteolytic potential of different polyethylenes developed for use in hip prostheses in preclinical studies. Also, previous studies, conducted by the same authors, using primary human peripheral blood mononuclear (in vivo method) cells had revealed a great deal of variation in the level of cytokines produced in response to UHMWPE particles between individuals [17]. However, the study by Green et al. [8] avoided such discrepancies in data by conducting their experiment in vitro. The question that still remains to be answered is whether mediators like cytokines play a direct role in the particle stimulated bone resorption activity of macrophages in vitro.
Crosslinked UHMWPE and its performance against counterface roughness
Recently, techniques such as X-ray-irradiation, electron-beam-irradiation, and chemically alternating molecular structure using peroxides have been developed to strengthen polyethylene components and help eliminate the wear problem [19]. Cross-linking refers to increasing the number of transverse connections to the long chain-like molecules in the UHMWPE plastic. An early study by Kurtz et al. [20] discussed the development and properties of crosslinked UHMWPE, which he called a “promising alternate biomaterial for total joint replacements.” The study stressed the need for additional studies on the subject, particularly the relationship between counterface roughness and the wear of crosslinked UHMWPE. Research performed by Saikko et al. [21] analyzed the wear behavior of electron-beam-crosslinked UHMWPE using a circularly translating pin-on-disk device. The Cobalt-Chromium (CoCr) metal counterfaces were either polished or roughened so that they represented the type of roughening and the range of surface roughness values (R = 0.014-0.24 microns) observed in explanted femoral heads of total hip prostheses. This study clearly demonstrated that there is a strong correlation between the counterface surface roughness R a and wear rate of UHMWPE. Crosslinking improved wear resistance considerably. Increasing the crosslinking radiation dose resulted in a more wear resistant polyethylene. For the baseline crosslinked polyethylene, the wear rate was 36.9 mg in a million cycles (mg/Mcycles.) The wear rate decreased to 9.0 mg/Mcycles when five times more radiation was applied, and further decreased to 1.1 mg/Mcycles when ten times more radiation was applied to the polyethylene. Additionally, the wear of crosslinked polyethylene against the roughest counterfaces was lower than the wear of conventional (not crosslinked) polyethylene against polished surfaces. Figure 5 illustrates the comparison of wear performance of these materials [21]. Also, the surface area and volume of wear particles generated during the tests decreased with increasing radiation dose [19, 20, 21]. Each one of the 24 points on the diagram represents an independent wear test of 5 weeks’ duration and six periodic wear measurements. You can see by the figure that crosslinked polyethylene shows an increase of wear factor with an increase of surface roughness. This tells us that crosslinking can improve wear resistance by decreasing the surface roughness of a crosslinked polyethylene [21].
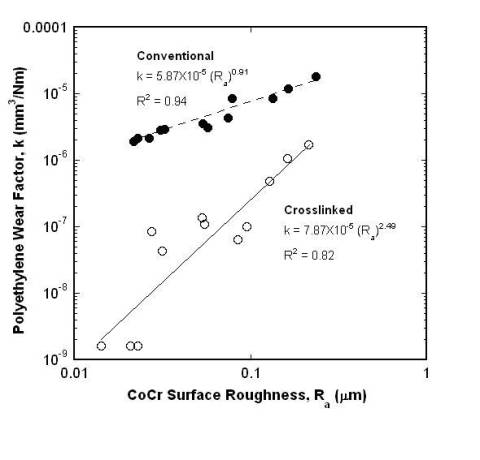
Figure 5 . Comparison of wear performance of conventional polyethylene versus UHMWPE at different CoCr surface roughness levels. Data was obtained from: Saikko V, Calonius O, Keranen J. Effect of counterface roughness on the wear of conventional and crosslinked ultrahigh molecular weight polyethylene studied with a multi-directional motion pin-on-disk device. J Biomed Mater Res 57: 506-512, 2001 [21].
The study by Saikko et al. not only expanded the earlier research by Kurtz et al. but provided new understanding of the effectiveness of crosslinking UHMWPE on wear. It is reasonable to assume that the tissue reaction would be less severe if electron-beam-crosslinked UHMWPE is used instead of the conventional polyethylene, because the amount of wear particles generated would be reduced. What is not known, however, is whether the wear particles sizes or their shape characteristics from irradiated crosslinked UHMWPE promote or lessen the degree of osteolysis.
DISCUSSION
The goal of this review has been to summarize state-of-the-art approaches to understand UHMWPE wear particles in total hip arthroplasty. It can be said that the recent approaches that have been used to study UHMWPE particle size such as the employed macrophage isolation and contact stress tests provide some understanding of the importance of particle size in causing Osteolysis in Total Hip Anthroplasty. It is imperative that these areas of study be developed to further clarify and understand the wear of UHMWPE.
Based on the research on particle size, it is clear that the largest and smallest particles failed to evoke any osteolytic response [1,8,7]. With this information, engineers may be able to design and produce a polymer material that produces particles in such “critical” size ranges. It has become clear, that particle shape, size, and morphology play a clear role in the onset of biological reactions within the body.
The study of material parameters and their impact on particle size provides some interesting results that concern various dynamics of particle size. The study by Landry et al. developed a technique to quantitatively measure the size and shape of UHMWPE wear particles and used this system to determine how different wear parameters affect the size and morphology of particles. The resulting figures that show the increase of the mean size of wear particles with the doubling of contact stress are consistent with the figures from in vivo sources [22,23]. Because particles generated from the different orientations had different sizes and shapes, the orientation as well as other material parameters also play roles in wear and particle generation. Although the investigation of material parameters and contact stress suggested that there are potential relationships between particle geometry and particle wear, it does not necessarily prove that particle size is related to surface energy [15]. These findings could be extremely critical to implant design, and further research should be conducted to either dispute or support and validate such correlations.
Because crosslinking illustrates that wear resistance can be enhanced greatly, the potential for market success for an electron-beam-crosslinked polyethylene cup is very high. Because the study by Saikko et al. is one of the first experiments on the vast subject of surface roughness, it would be very interesting to see clinical studies further examine the correlation between counter surface roughness and particle size.
Problems still remain in conducting certain experiments and analyzing the results in the broad area of osteolysis. According to Ingham et al [13], there are discrepancies in data from the use of “non-validated cell lines that do not respond in a manner that is similar to primary macrophages.” Moreover, in vitro studies are crucial in studying particles under controlled circumstances, but they bring attention to fact that many investigators have overlooked the importance of using sterile endotoxin-free particles in their in vitro assay systems, which may result in false positive results [24, 25, 26, 27]. To increase the efficacy of research, the in-vitro methods require great attention and development.
SUMMARY
It appears that the amount of research that needs to be done to have a clear understanding of the role of UHMWPE wear and its role in limiting the life of Total Hip Anthroplasty is significant. The final arbiter for the present innovations in UHMWPE will be the clinical proving ground. With in-depth knowledge of how particles are actually formed and which kind of particles cause osteolysis, the longevity of implants in THA can be clearly extended. Patients, both the young and elderly will have the most need in this biomedical area. Further, future generations can benefit greatly from the implant experiences and trials conducted now. The emphasis should not be placed on the volumetric wear of the prosthesis, but the number of particles generated within the biologically active size range for a given wear volume. This area of focus will determine the duration of survival of a hip implant. Therefore, understanding, controlling, and consequentially mitigating the wear of UHMWPE in implant systems is undoubtedly of prime importance in biomaterial research in the years to come.
REFERENCES
1) Green T, Fisher J, Matthews B, Stone M, Ingham E. Effect of size and dose on bone resorption activity of macrophages by In vitro clinically relevant ultrahigh molecular weight polyethylene particles. J Biomed Mater Res (Appl Biomater) 53: 490-497, 2000
2) Medical Multimedia Group.
3) Charnley J. The lubrication of animal joints. Institution of Mechanical Engineers: Symposium on Biomechanics 1959; 17: 12-22.
4) Good V., Clarke I., Gustafon A., Anissian L. Wear of UHMWPE an PTFE in a him simulator: comparison of fluid volume and protein concentration and anatomical vs. inverted positioning. In 47 th Annual Meeting, Orthopaedic Research Society, 2001, p. 1015
5) Konttinen Y. T., Xu J. W., Patiala H., Imai S., Waris V., Li T.F., Goodman S.B., Nordsletten L., Santavirta S. Cytokines in aseptic loosening of total hip replacement. Curr Ortho 1997; 11:40-47
6) Landry M, Blanchard C, Mabrey J, Wang x, Agrawal C. Morphology of In vitro generated wear particles as a function of contact conditions and material parameters. J Biomed Mater Res (Appl Biomater) 49: 61-69, 1999
7) Savio J, Overcamp L, Black J. Size and shape of biomaterial wear debris. Clin. Mater., 1994, 12: 1-47.
8) Green TR., Fisher J, Stone MH, Wroblewski BM, Ingham E. Polyethylene particles of a “critical size” are necessary for the induction of cytokines by macrophages in vitro. Biomater 1998; 19: 2297-2302
9) McGee M. A., Howie D. W., Neale S. D., Haynes D. R., Pearcy M. J. The role of polyethylene wear in joint replacement failure. Proc Inst Mech Eng Part H., 1997; 211:65-72.
10) Surin, V. “Polyethylene for Total Hips.” (10 Apr. 1999): 8pp. On-line. Internet .24 February 2003. Available FTP: http://www.totaljoints.info/polyethylene_for_total_hips.htm
11Charnley, J. Fracture of femoral prostheses in total hip replacement. A clinical study. Clin.Orthop., 1975, 111, 105-120
12) Wilert, H. G. and Semlitsch, M. Reactions of the articular capsule to wear products of artificial joint prostheses. J. Biomed. Mater. Res., 1977, 11, 157-164.
13) Ingham, E. and Fisher, J. Biological reactions to wear debris in total joint replacement. Proc. Instn Mech. Engrs, Part H: J. Engineering in Medicine, 2000, 214 (H1), 21-37
14) Wilert H. G., Bertram H, Buchhorn G. H., Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Rel Res 1990; 258: 95-107
15 ) Elfick AP, Green SM, Krikler S, Unsworth A. The nature and dissemination of UHMWPE wear debris retrieved from periprosthetic tissue of THR. J Biomed Mater Res, 2001, 65A(1): 95-108
16) Voronov I, Santerre J. P., Hinek A, Callahan J.W., Sandhu J, Boynton E. L., Macrophage phagocytosis of polyethylene particles in vitro. J Biomed Mater Res 39: 40-51
17) Murray D. W., Rushton N., Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg 1990; 72B: 988-992
18) Howie AW, Haynes Dr, Rogers SD, McGee MA, Pearcy MJ. The response to particulate debris. Ortho Clin N Am 1993; 24: 517-524
19) Oonishi H, Takayama Y, Tsuji E. Improvement of polyethylene by irradiation in artificial joints. Radiat Phys Chem 1992; 39: 495-504
20) Kurtz S.M., Orhun K. M., Evans M., Avram A.E.. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint anthroplasty. Biomaterials 20: 1660-1668, 1999
21) Saikko V, Calonius O, Keranen J. Effect of counterface roughness on the wear of conventional and crosslinked ultrahigh molecular weight polyethylene studied with a multi-directional motion pin-on-disk device. J Biomed Mater Res 57: 506-512, 2001
22) Matthews J.B., Green T.R. Store M. H., Wroblewski B. M., Fisher J, Ingham E. Production of osteolytic mediators by primary human macrophages in response to stimulation with clinically relevant UHMWPE particles in vitro. Oxford: Proc Brit Ortho Res Soc; 1998. p 6.
23) Revell PA. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng J Eng Med 1997; 211 H: 187-197.
24) Shanbhag A. S., Bailey HO, Hwang D. S, et. al. Chemical and morphological charachterization of wear debris associated with acetabular screw holes. Transactions of the 21 st Annual Meeting of the Society for Biomaterials. San Francisco, CA; 1995: 325
25) McKellop HA, Campbell P, Park SH, et al. The origin of submicron polyethylene wear debris in total hip anthroplasty. Clin Orthop Rel Res 1995; 311: 3-20
26) Bi, Y., Van Der Motter, R. R., Ragab, A. A., Goldberg, V.M. and Green field, E.M. The role of endotoxin in stimulation of osteoclast differentiation by orthopaedic wear particles. In Processings of the 45 th Annual Meeting of the Orthopaedic Research Society, 1999, p. 5.
27) Cho, D.R., Hong, C.Y., Baran, G.R. and Goldring, S.R. Adsorbed endotoxin mediates differential effects on particle-induced stimulation of cytokine and chemokine release. In Processings of the 45 th Annual Meeting of the Orthopaedic Research Society, 1999, p.6.